Raman Spectroscopy
A technological breakthrough often starts with a theory which is waiting for a proof. In the case of Raman spectroscopy, which is now a vital tool in life sciences and materials analysis, it was an insight by a scientist who spent his entire professional life in his home country.
Chandrasekhara Venkata Raman (Image courtesy of From Nobel Lectures, Physics 1922-1941, Elsevier Publishing Company, Amsterdam, 1965
Chandrasekhara Venkata Raman was born in 1888 in Tiruchirapalli, in India. After studying at the University of Madras, he was encouraged to continue his research in England, but was prevented by poor heath. Instead, he remained in India, working in the civil service until he was appointed to the post of Palit Professor of Physics in Calcutta in 1914.
In 1930, Raman became the first Asian to receive a Nobel Prize for Physics. The subject was his research into the scattering of light. Raman had always taken a deep interest in the behaviour of waves, studying subjects as diverse as the vibration of piano strings and the cause of the blue colour of sea water.
Two years earlier, Raman had been investigating how light interacts with materials at the molecular level. He was studying the work of another scientist, Adolf Smekal, who had hypothesised that light could be scattered by materials in such a way that the properties of the light are changed.
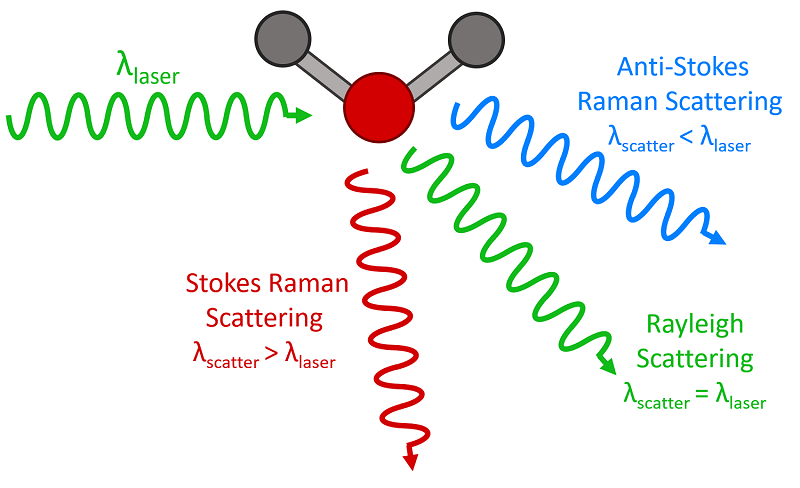
Scattering is the term which describes how light interacts with a material, and where the light path deviates from a straight line. The blue colour of the sky is caused by the scattering of blue light, by the tiny gas molecules in the atmosphere. This is called Rayleigh scattering – this scattering causes the blue light to be redirected, making it appear to come from all parts of the sky. As a result, when we look up, we see blue light coming from every direction, giving the sky its characteristic blue colour. The intensity of scattered light is directly proportional to 1/ λ⁴. This means that shorter wavelengths, such as blue and violet light, are scattered more effectively than longer wavelengths (e.g., red or yellow), which is why we see the sky as blue during the day. The wavelength or colour of the light does not change after encountering the molecule; it is simply redirected.
Blue sky at Torres del Paine, Chile.
While the majority of incident light undergoes elastic scattering, in which most light photons are scattered with their initial energy, a fraction of photons experience inelastic scattering, known as Raman scattering. In this case, when a photon interacts with a molecule, it can excite the molecule's vibrational modes. As a result, some of the photon's energy is transferred to the molecule, causing the scattered photons to be emitted with lower energy. This lower energy corresponds to a longer wavelength, resulting in a shift toward the red end of the electromagnetic spectrum.
Three types of scattering processes that can occur when light interacts with a molecule. Image courtesy of Edmund Optics
Importantly, the amount of shift is precisely related to the molecular composition of the material. So, if the Raman shift can be measured precisely, the material can be analysed with a beam of monochromatic light.
The spectrum appears as a faint series of lines alongside the original wavelength, each line corresponding to a peak in the spectrum indicating a different molecule. By measuring the wavelength difference and intensity of each peak relative to the stronger Rayleigh peak, the concentration and type of each molecule can be determined. By resolving and projecting the spectrum onto a light-sensitive plane, the peaks corresponding to the Raman shift can be measured in an image. In the early days, gas discharge lamps, such as mercury or sodium lamps, were used with diffraction gratings and photographic film, with exposures lasting several hours.
As so often happens in engineering, it took inventions in parallel fields to enable the widespread success of Raman spectroscopy. The first invention was the laser, develop during the 1950s, and providing a powerful source of perfectly monochromatic light. The second invention was the CCD image sensor in 1969, which provided higher sensitivity and faster response than film, which was later replaced by the CMOS sensor in the late 1990s. The third development was computer image processing, which provided a method of extracting the Raman signal from the background noise. Now, with a precise light source, and a sensitive detector for the spectral lines, coupled with digital image enhancement, a fast Raman instrument could be created, and the true potential of this technique could be realised.
In rapid point-of-care analysis of tissue and blood samples, biomolecules give a good Raman response due to the properties of the complex organic molecules, so that ‘fingerprints‘ of biomarkers can be clearly recognised. Blood glucose concentration can be measured in-vivo, replacing regular invasive blood sample extraction. Drug and toxin concentrations, even cancer indicators, can be measured from blood and urine samples.
The Raman technique is highly versatile. It can measure anaesthetic and respiratory gas compositions during surgery. It can even be used to measure the temperature of a substance. It has been used to measure micro-stresses in semiconductor wafers.
ISDI’s SE1K128 image sensor was developed as a custom design project for RSP Systems (Denmark). Engineered for low dark current noise and high Quantum Efficiency (QE) in the NIR region, this sensor is specifically tailored to supports RSP Systems' handheld and wearable applications. More details about this custom design project will be available in our upcoming Case Study on the development of the SE1K128 sensor with RSP Systems.